58 Which of the Following Is an Acidic Oxide
When reacting with water these compounds form oxacid acids but if they are in the. Classify the following into acidic oxides and basic oxides.
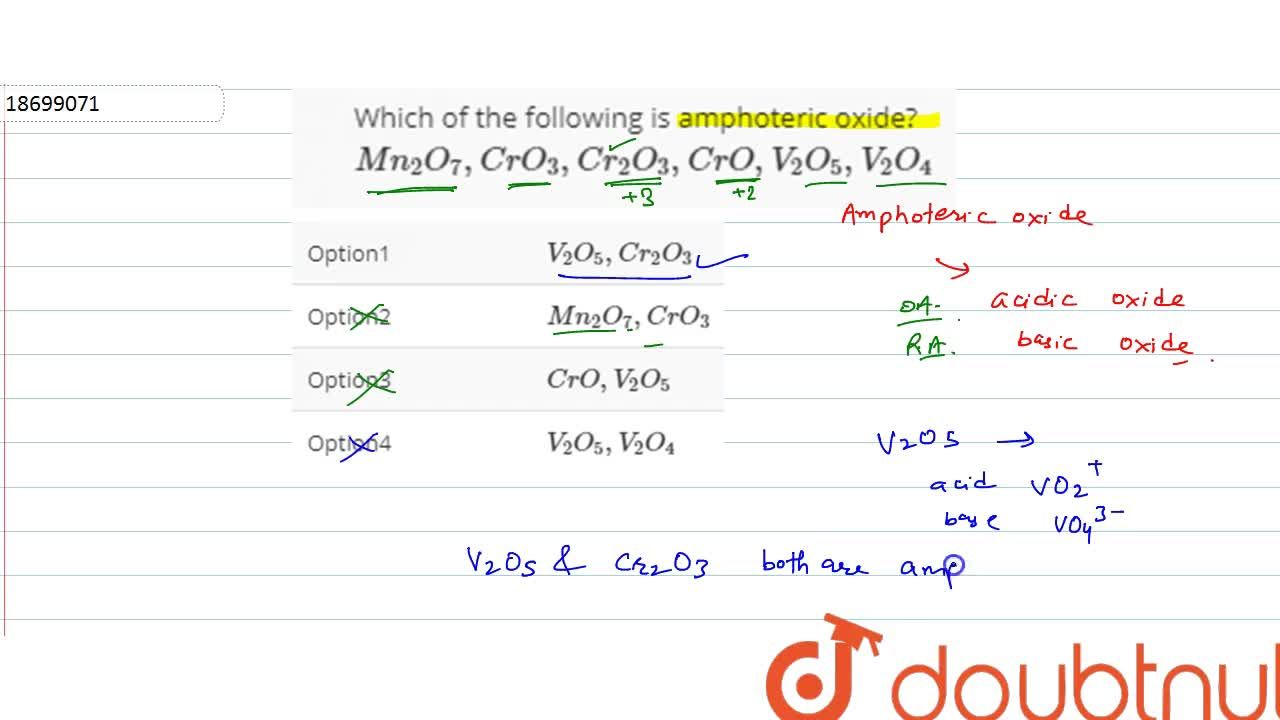
Which Of The Following Is Amphoteric Oxide Mn 2 O 7 Cro 3 Cr 2 O 3 Cro V 2 O 5 V 2 O 4
AP4O6 BP4O10 CAs2O6 DAs4O10 - 18136907.

. Underset underset less basic More acidic underbrace B_ 2O_ 3 underset Amphoteric underbrace Al_ 2O_ 3 and Ga_ 2O_ 3 underset Basic underbrace ln_ 2O_ 3 and Tl_ 2O_ 3. Which of the following oxide is more acidic. Like because murder have more power to echo the electron density.
Which of the following oxide is least acidic. Most acidic oxide is- MgO CaO Na2O Al2O3. Neutral oxides- CON_2O etc.
Non-metals react with oxygen to form acidic compounds of oxides which are held together by covalent bonds. Most acidic oxide among the following isa. These compounds can also be called as acid anhydrides.
I an acidic oxide ii a basic oxide. Cl2O7Like the video and subscribe the channel for more learning videos. The acidic character of the oxides decreases.
Acidic oxides are oxides that react with water to form an acid or with a base to form a salt. Amphoteric oxides- Al_2O_3ZnOPbO etc. So we are going to discuss which magnesium oxide will be acidic oxide.
Acidic character of oxides decreases down the group. 5 is more acidic than A s 4 O 6 OS. C l 2 O 7.
Experts are tested by Chegg as specialists in their subject area. Acid anhydrides usually have a low melting and boiling point except for compounds like B 2 O 3 and SiO 2 which have high melting points and form giant molecules. Thus A s 4 O 6 is least acidic.
Thus A S 4 O 10 OS. The oxide which will form acid on reacting with water will be acidic in nature. P 4 O 10.
Thus oxides of As are less acidic than those of P. Which of the following is an acidic oxide. Who are the experts.
Na2O SO2 MgO CO2. Acidic oxides- SO_2CO_2NO_2 etc. An acidic oxide is an oxide which when combined with water gives off an acidA basic oxide is an oxide which when combined.
What are called acidic oxides and basic oxides. CO H 2 O H 2 C O 2. Theres edge plus can be released very.
Ln_ 2O_ 3 Answer. Ai Oxide of non-metals which dissolve in water to form acidic solution. Correct option a As4O6.
Which one among the following is an acidic oxide. Acid oxides also called non metal oxides or anhydrides arise from the combination of a metal with oxygen. Which of the following term best describes the nature of zinc oxidesa An acidic oxide b A basic oxidec An amphoteric oxide d A neutral oxide Asked on 21st Dec 2020 aWith the help of examples describe how metal oxides differ from non-metal oxidesbWhich of the following elements would yield.
Most non-metals on the RHS of the PT form acidic oxides excluding noble gases which do not form any oxides and a few elements such as Al Sn Zn Pb which form amphoteric oxides. Acidic character increases with increase in oxidation state. This shows that CO is not acidic in nature.
Acidic strength of the except increase as the oxidation state of the central accountability increase. The acid oxides also called non-metallic oxides or anhydrides arise from the combination of a non-metal with oxygenSince the electronegativity difference between these elements is low the bonds that form between them are covalent. Since the difference in electronegativity between these elements is low the bonds that are formed between them are covalent.
Which of the following is an acidic oxide. The most acidic oxide among the following is. When CO carbon monoxide reacts with water hydrogen gas is released and carbon dioxide gas is also formed.
1 point a na2o b co c co2 d al2o3 2 See answers Advertisement. AnsweredDec 24 2018by ranik674kpoints selectedDec 24 2018by faiz. The number of acidic oxides oxides among the following are CO_2Rb_2OMn_2O_7CrO_3Na_2OMgOAl_2O_3SO_2 Arrange the following oxides in decreasing acidic order SiO_2.
1 point a na2o b co c co2 d al2o3 - 20901061 itzShanaya itzShanaya 13082020 Chemistry Secondary School answered Which one among the following is an acidic oxide. The acidic nature of oxides changes from acidic to basic through amphoteric on moving down the group. Hello portion is this.
They are usually oxides of either nonmetals or sometimes of metals in. Arsenic oxide carbon dioxide tellurium oxide. Therefore BeO is the most acidic in nature as the acidity of oxides decreases with an increase in the electropositive character of the central atom.
CO is a neutral oxide which means it neither behaves as an acidic oxide not basic oxide. Cr 2 O 3. When reacting with water these compounds form oxacid acids but if they are in the presence of hydroxides what is.
The correct option is C C l 2 O 7 Oxides of metals are basic in nature oxides of non-metals are acidic in nature whereas the oxides of metalloids are amphoteric in nature. Basic oxides- CaONa_2OK_2O etc. Which of the following is an acidic oxide.
The acidity of these oxides decreases down each group as the elements become more metallic in character. We review their content and use your feedback to keep the quality high. Fe 2 O 3.
The higher the oxidation states the more acidic SO3 SO2.

Sample Paper Mcq The Table Given Below Shows The Reaction Of A Few

Oxide Nonmetal Oxides Britannica

X Y And Z Are The Three Elements Each One Belongs To Any One Of The Group Ia Iiia And Va The Oxide Of X Is Amphoteric The Oxide Of Y Is

Which Of The Following Oxide Is Most Acidic

Which Of The Following Oxide Is Amphoteric

Surface Processing For Iron Based Degradable Alloys A Preliminary Study On The Importance Of Acid Pickling Sciencedirect

Among The Following The Most Basic Oxide Is
Is Silicon Oxide Acidic Or Basic Quora

Case Base Mcq The Reaction Between Mno2 With Hcl Is Depicted In

Ncert Exemplar Class 10 Science Solutions Chapter 3

Which Of The Following Oxides Of Chromium Is Amphoteric In Nature
When Al2o3 Is Acidic And When Is Basic Oxide Quora

Amphoteric Oxide Definition Identification Examples Videos And Faqs Of Amphoteric Oxide

Which Of The Following Oxides Is Not Expected To React With Sodium Hydroxide

What Are Oxides Give Two Examples Of Each Of The Following Oxides A Basic Oxide B Acidic Oxide C Amphoteric Oxide D Neutral Oxide
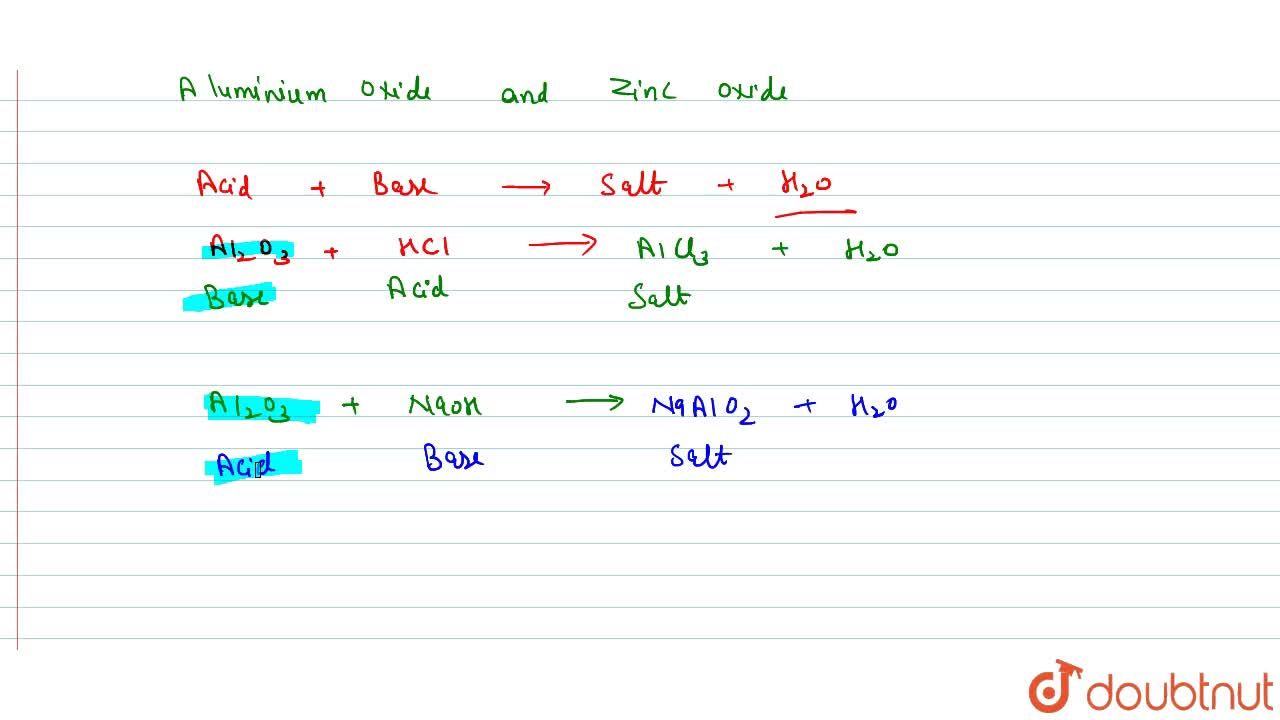
What Are Amphoteric Oxides Give Two Examples Of Amphoteric Oxides

Neet 2018 Qp With Solutions Code Aa Part 15 Question Paper Coding Neet Exam

Comments
Post a Comment